In Any Chemical Process Matter Is Neither Lost Nor Gained
A chemical change in which a single compound is broken down into two or more simpler products. In physics and chemistry the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy the mass of the system must remain constant over time as the systems mass cannot change so quantity can neither be added nor be removed.

Discovering The Link Between Nutrition And Skin Aging Disease Disorders Cardiovascular
In any chemical process matter is neither lost nor gained.

. In any chemical process matter is neither lost nor gained. Put another way the First Law of Thermodynamics states. Energy is neither created nor destroyed.
22 Evolution of Atomic Theory. The First Law of Thermodynamics. These electron-transfer reactions are termed as oxidation-reduction reactions or Redox reactions.
Therefore equations depicting reactions must be balanced. These rules are referred to as laws of chemical combination. In any chemical process matter is neither lost nor gained.
That is same with chemical equation. This is a measure of the quantity of matter. Oxidation and Reduction reactions- The chemical reactions which involve the transfer of electrons from one chemical substance to another.
The breaking of bonds which always absorbs energy and increases mass. The Law of Conservation of Atoms in a chemical reaction states that atoms are neither gained nor lost in the process they just get rearranged Its too late for the dog to have its dinner. Decomposition Reaction A chemical change in which a single compound is broken down into two or more simpler products.
There are four general types of basic reactions. A chemical change that involves an exchange of positive ions between two compounds. Energy Can Neither Be Created Nor Destroyed.
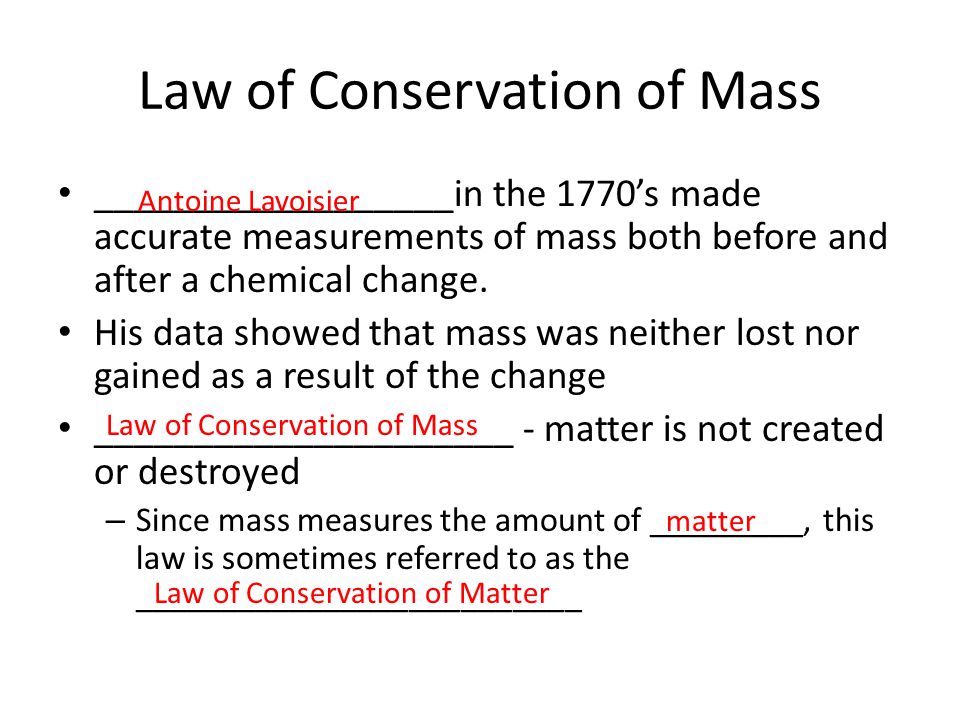
Recall again when we talked about Lavoisier when he showed that in chemical reactions mass is neither lost or gained. This is a change in both form and nature of substance in which bonds are broken. The Law of Conservation of Mass states that matter can neither be created nor destroyed in a chemical reaction.
Fire is a conversion of chemical energy into thermal and electromagnetic energy via a chemical reaction that combines the molecules in fuel wood. Therefore the quantity of mass is conserved over time. There are no more atoms at the end of the chemical reaction than there were at the beginning.
The loss or gain of mass during all reactions whether chemical or nuclear is very well established and has been confirmed experimentally. Ions are charged atoms that form when an atom donates or accepts one or more negatively charged electrons. Due to the stable configuration the noble gas atoms neither have any tendency to gain or lose electrons and therefore their combining capacity or valency is zero.
During any chemical change atoms are neither created nor destroyed. Chemical energy seems to be the last living form of energy so a corpse no longer able to process the energy transfer goes into a default mode assumption where it becomes something but no one knows another assumption is the brain still functional throughout the decomposition process and if not whos in charge of overseeing a correct. Samples of a particular compound all have the same elemental proportions by mass.
This is a solid that form during a chemical reaction typically in solution. Mass is never lost nor gained - neither. Internal Energy E is the sum of all kinetic and potential energy of all components of the system translational rotational vibrational electronic nuclear etc.
If energy is lost by the system then it. Cations ions with a positive charge are attracted to anions ions with a negative charge. Its impossible to know how far and through what.
It deals with the total amount of energy in the universe and in particular it states that this total amount does not change. That is the same number of atoms of each kind must appear on opposite sides of. They are so inert that they even do not form diatomic molecules and exist as monoatomic gaseous atoms.
In covalent bonds the participating atoms do not lose or gain electrons but rather share them. This attraction is called an ionic bond. When two elements form different compounds a given mass of one element will combine with masses of the other element in a small whole-number ratio.
The First Law of Thermodynamics. This law states that energy is neither created nor destroyed. For every apostrophe omitted from an its there is an extra one put into an its.
Any heat interaction that takes place in the system with its surroundings also changes its internal energy. The Law of Conservation of Mass states that matter is neither created nor destroyed in a chemical reaction We are following this idea when we balance our equations by being. Is matter never lost or gained according to the law of conservation of matter in a chemical reaction.
In simple terms this law states that. Truss states The Law of Conservation of Apostrophes as follows. In reactions under normal laboratory conditions matter is neither created nor destroyed and elements are not transformed into other elements.
He proved that the mass of the products in a chemical reaction is equal to the mass of the reactants. But since energy remains constant from the first law of thermodynamics the total change in internal energy is always zero. Any energy lost by the system is gained by the surroundings.
A chemical change that involves an exchange of positive ions between two compounds. The oxidation and reduction reaction also involve the addition of oxygen or hydrogen to different substances. The first law of thermodynamics thinks big.
In any chemical process matter is neither lost nor gained. Law of Conservation of Mass. In any physical or chemical change matter doesnt appear or disappear.
There are five basic laws of chemical combination that govern the chemical combinations of elements. The formation of bonds which always releases energy and decreases mass. Atoms created in the stars a very very long time ago make up every living and nonliving thing on Eartheven you.

Law Of Conservation Of Energy Video Khan Academy

Pdf Dissolving The Dichotomies Between Online And Campus Based Teaching A Collective Response To The Manifesto For Teaching Online Bayne Et Al 2020
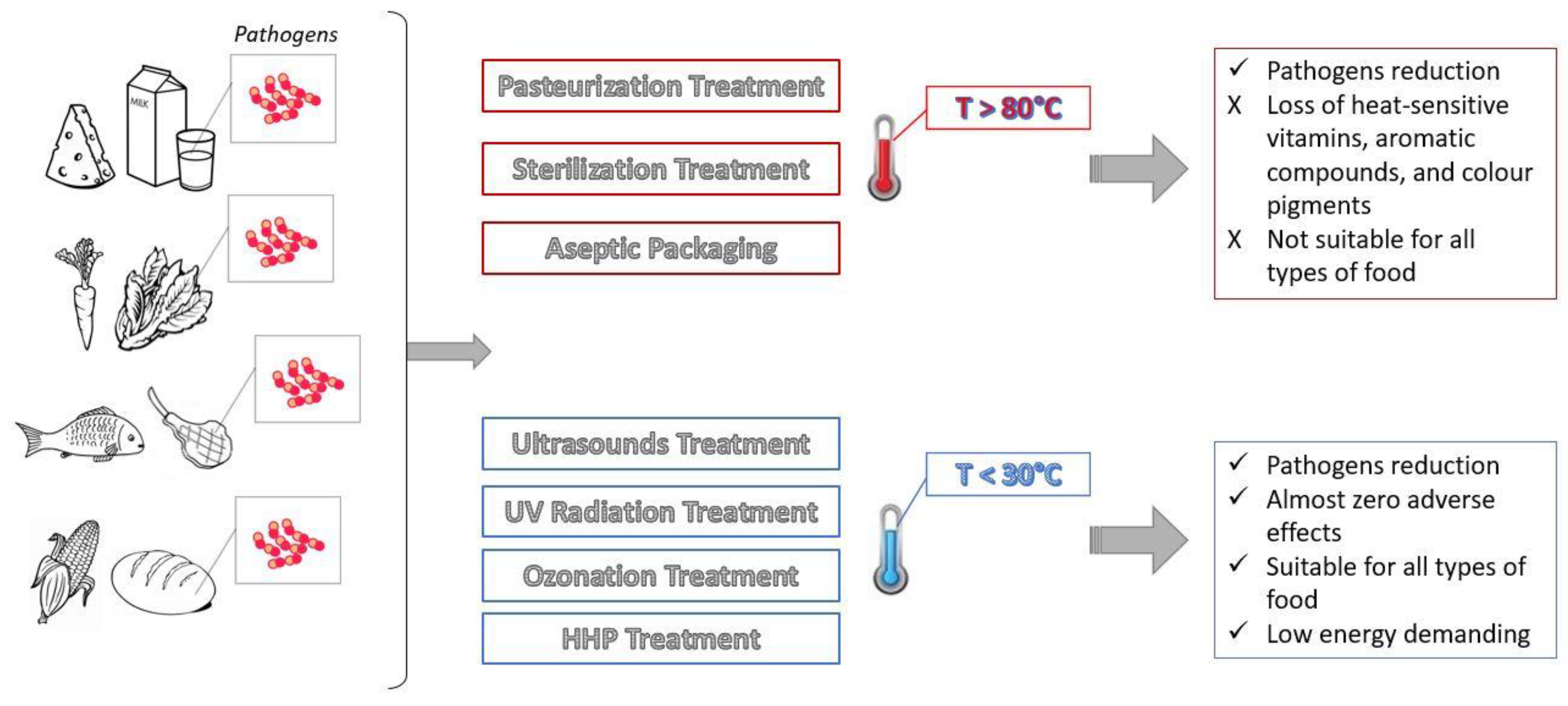
Applied Sciences Free Full Text Advances Applications And Comparison Of Thermal Pasteurization Sterilization And Aseptic Packaging Against Non Thermal Ultrasounds Uv Radiation Ozonation High Hydrostatic Pressure Technologies In Food

Pdf Muography And Its Potential Applications To Mining And Rock Engineering
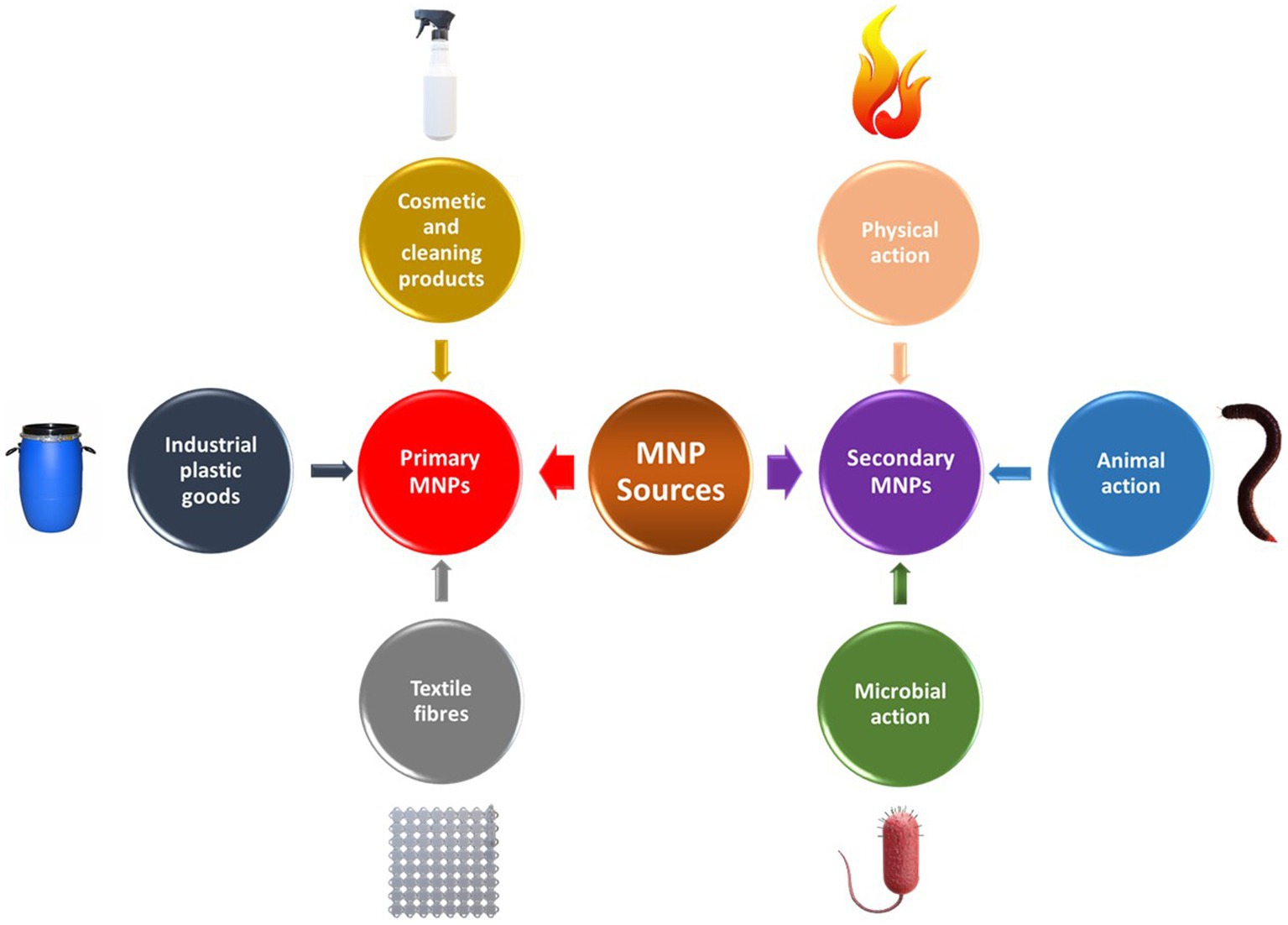
Frontiers Environmental Impacts Of Microplastics And Nanoplastics A Current Overview Microbiology

Chemical Equations A Chemical Equation Is A Form Of Shorthand Which Gives An Outline Of The Progress Of A Chemical Reaction 2 H 2 O 2 H 2 O 2 Reactant Ppt Download
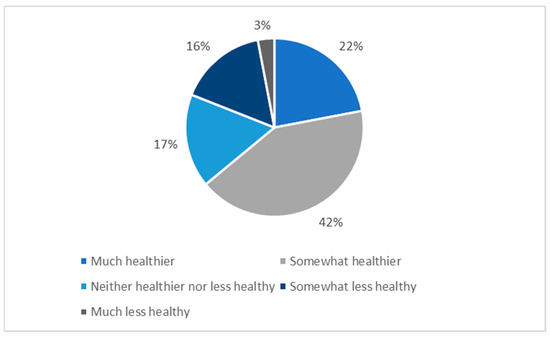
Nutrients June 2021 Browse Articles

Mariupol Mayor Cites Thousands Dead Says Complete Evacuation Needed
This Article Won T Change Your Mind The Atlantic

Traces Of Ancient Life Tell Story Of Early Diversity In Marine Ecosystems Study School Of Medicine Analysis
Introduction To Matter 8th Grade Science Ppt Video Online Download

Pages 3 To 33 Quantum Chemistry Target Completion Date October Ppt Download

Global Volcanism Program Krakatau

Chemical Reactions Test Review Quizizz

Chapter 4 Fire Behavior Ppt Download

Pages 3 To 33 Quantum Chemistry Target Completion Date October Ppt Download

Carbon Compounds Why Is Carbon The Basis For Life It Has 4 Electrons In Its Outer Valence Electron Shell Octet Rule The Most Stable Elements Have Ppt Download

Comments
Post a Comment